|
Vitamin C (Ascorbic acid)
Ascorbic acid is a 6-carbon lactone whose biosynthesis occurs in the liver of mammals with the exception of guinea pigs, primates (apes and monkeys) and man (1). Ascorbic acid biosynthesis is absent in invertebrates,
insects and fishes. In mammals, the synthesis of ascorbic acid starts from glucose and it involves nine steps.
For those interested, the biosynthesis of ascorbic acid in plants is shown
on this page.
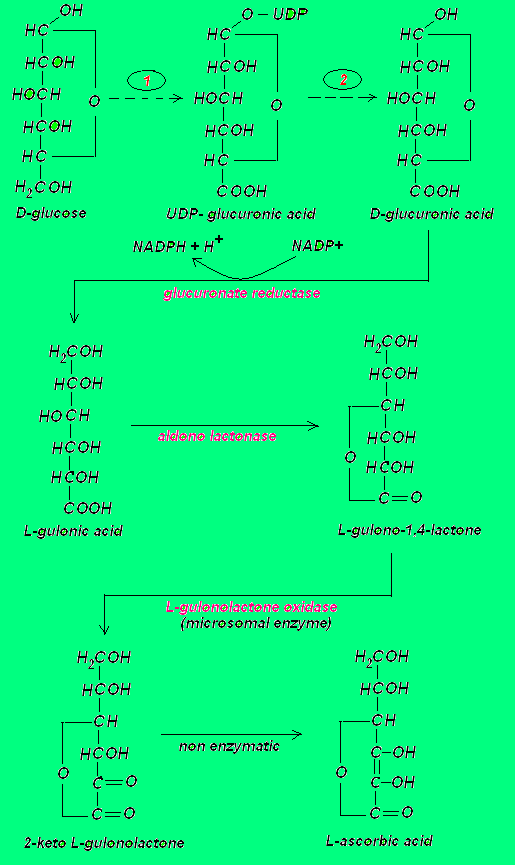
In the diagram above only the main steps are shown. As part of the first broken arrow the following reactions occur:
D-Glucose ---> Glucose-6-P ---> Glucose-1-P ---> UDP-Glucose ---> UDP-D-glucuronic acid. As part of the second broken arrow the UDP-D-glucuronic acid is converted to D-glucuronate-1-P, which upon hydrolysis yields D-glucuronic acid.
It appears that some 25 million years ago a mutation occured in the gene that codes for the enzyme that converts L-gulono-1,4-lactone into the immediate precursor of vitamin C, namely 2-keto L-gulono-1,4-lactone (1). Thus,in order to survive, humans have to get the necessary vitamin C from their diet.
Ascorbic acid is an electron donor and therefore a reducing agent. It can be looked upon as a diacid whose pKs are 4.1 and 11.8, respectively. However, at the blood plasma pH of 7.4 over 99% of vitamin C is present as
ascorbate(AscH-). By donating electrons the ascorbic acid prevents sensitive biological molecules from becoming oxidized thus acting as an antioxidant. These electrons are lost sequentially with the formation
in the first step of an ascorbyl radical, which upon the loss of another electron turns into dehydroascorbic acid. The formation of these molecular species in biological systems is mediated by a wide range of oxidants, such as oxygen and its reactive species (superoxide, hydroxyl radical), HClO, NO. and the transitional metals iron and copper. In both mammalian and plant systems, some of the oxidized ascorbic acid is converted back to its reduced form by dehydroascorbate reductase using NAD(P)H/GSH as hydrogen donor (2, 3). The rest is degraded and excreted.
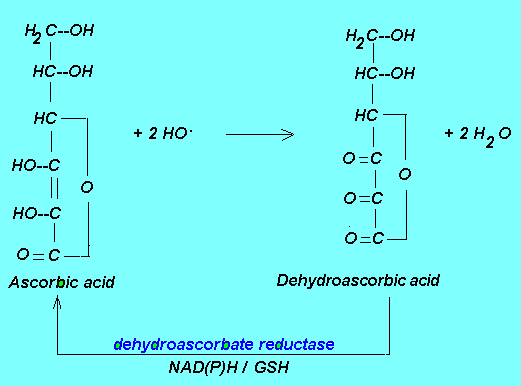
For more on the chemistry of ascorbate and its antioxidant chemistry the reader is encouraged to look
at this short presentation.
Below, some of the processes in which the ascorbic acid is involved are listed:
- Essential for collagen synthesis. Collagen is the main component of cartilage, tendons and connective tissue. To see how vitamin C is involved in collagen synthesis click on the
Next button.
- Essential to the immune function by enhancing white blood cells activity and interferon levels.
- Acts as a general free radical scavenger in the body's aqueous environment, inside and outside the cells. Works synergistically with other antioxidants such as vitamin E and glutathione. Thus, the a-tocopheroxyl
radical (generated when oxidants interact with the a-tocopherol-containing plasma LDL) is reduced back to a-tocopherol.
- Involved in the absorption of iron by reducing ferric to ferrous iron in the intestinal tract.
- Acts as co-factor for the hydroxylation reaction in carnitine biosynthesis.
- Acts as co-factor for dopamine b-hydroxylase, which catalyzes the conversion of
dopamine to norepinephrine.
- Involved in enzymatic reactions (amidation) that yield biologically active oxytocin, vasopressin, cholecystokinin and
a-melanotripin.
- Involved in the microsomal hydroxylation of cholesterol in the pathway that converts cholesterol into bile acids.
- Involved in the conversion of the amino acid tryptophan into 5-hydroxytryptophan, a precursor of serotonin.
- Involved in the conversion of p-hydroxyphenylpyruvate (an intermediate in the phenylalanine degradation pathway) to homogentisate.
- As mentioned on the Generation of Free Radicals page ascorbate can generate reactive
oxygen species (ROS) through an "auto-oxidation" reaction. Since molecular oxygen cannot
react directly with most biomolecules because of spin restriction it became apparent
that transition metal ions such as copper, which are redox catalysts can catalyze
the reaction of molecular oxygen with ascorbate. Both ROS and ascorbyl radical are
formed in the reaction (4). This may have biological as well as medical implications.
Thus, it was shown that plasmin and other serine proteases were inactivated by the
ascorbate-copper couple (5) and this may play a role in controlling the life span
of thrombi at sites of injury. Platelets and leukocytes, which are always present
at wound sites contain copper and ascorbate, respectively. Cellular toxicity caused
by the ascorbate-copper pair was demonstrated in bovine corneal endothelial cells
in vitro (6) and on sarcoma tumor cells in mice (7).
- Interactions: Vitamin C synergists: iron, selenium, nickel, germanium, vitamin A, bioflavonoids such as rutin and hesperidin.
For instance, bioflavonoids function synergistically with the ascorbate (forming
the so-called vitamin C complex)in scavenging ROS as well as in collagen synthesis
and supporting a healthy immune system (recall that leukocytes are rich in ascorbate).
Ascorbate and iron depend on each other for optimal absorption and selenium as part
of the antioxidant enzyme glutathione peroxidase supports the ascorbate in
scavenging the oxygen free radicals.
Vitamin C antagonists: zinc, manganese, calcium, copper (II), vitamin E.
High copper levels can deplete the ascorbate because as seen earlier the ascorbate-Cu2+
pair can generate ROS so additional ascorbate is required to scavange those reactive species.
High ascorbate intakes lower manganese levels and boosts insulin production, which
is bad for those with hypoglycemic tendencies that exhibit low sodium for sodium
slows insulin response. Low manganese also means decreased ability of the liver to
store glycogen. Prolonged high intakes of vitamin C can affect calcium metabolism
by lowering calcium stores, particularly the bones. However, high vitamin C intakes
may be beneficial for people with high calcium levels as wel as high levels of
copper and zinc for vitamin C can lower the body levels of these minerals. That is
why for healthy individuals a high intake of vitamin C may have harmful effects in
the long run because of these interactions. Because of their synergism a
higher vitamin C intake will require a higher vitamin E intake to balance the ratio
between these two vitamins. An imbalance between vitamin C and E in regard to the
trace mineral nickel can affect the vasodilating and vasoconstrictive properties of
the coronary arteries. This may be a problem for those suffering from angina-related
conditions. Aspirin was shown to interfere with ascorbate uptake by white cells.
- Health benefits: There have been suggestions that supplementation with doses above the RDA (recommended daily allowance of 100 mg of vitamin C) may be beneficial in several conditions, such as autoimmune disorders, bacterial and viral infections, cancer, capillary fragility, cardiovascular disease, fatigue, gum disease, skin disorders, etc. Clear signs of scurvy appear at vitamin C intakes below 10 mg. However, large controlled epidemiological studies have failed to show benefit. There is some evidence however, that ascorbic acid derivatives such as ascorbyl stearate inhibited the proliferation of certain human cancer cells by interfering with cell cycle progression and triggered apoptosis by modulating signal transduction pathways (9). For a review on the possible role of vitamin C in disease prevention see Ref. 8 & 9.
- Best food sources: fresh fruits and vegetables.

There is still a lot of debate among nutritionists as to what the optimum daily intake of vitamin C should be. Never in the history of research on the biomedical role of vitamins has there been so much debate raised as by vitamin C. To learn more about some of the less known aspects of the history of vitamin C research please go to
this page
References
1. Chatterjee, I.B.(1973) Science, 182, 1271-1272. Evolution and biosynthesis of ascorbic acid.
2. Choi, J.L. & Rose, R.C. (1989) Proc.Soc.Exp.Biol.Med. 190(4) 369-374. Regeneration of ascorbic acid
by rat colon.
3. Chen, Z. et al. (2003) Proc.Natl.Acad.Sci. USA 100(6) 3525-3530. Increasing vitamin C content of plants
through enhanced
ascorbate recycling.
4. Miller, D.M. et al. (1990) Free Radic.Biol.Med. 8(1) 95-108. Transition metals as
catalysts of "autoxidation" reactions.
5. Lind, S.E. et al. (1993) Blood 82(5) 1522-1531. Oxidative inactivation of plasmin
and other serine proteases by
copper and ascorbate.
6. Yu, H.S. et al. (1990) Curr.Eye Res. 9(2) 177-182. Ascorbate-enhanced copper toxicity on bovine corneal endothelial
cells in vitro.
7. Bram, S. et al. (1980) Nature 284(5757) 629-631. Vitamin C preferential toxicity
for malignant melanoma cells.
8. Padayatty, S.J., Katz, A., Wang, Y. et al.(2003) J.Am.Coll.Nutr. 22(1) 18-35. Vitamin C as an antioxidant: Evaluation
of its role in disease prevention.
9. Naidu, K.A. (2003) Nutr.J. 2:7 Vitamin C in human health and disease is still a mystery? An overview.
|



